CASE STUDY
Lightweight and Lifesaving: LMT’s MRI-Compatible Incubators Get 33% Lighter with AM
Discover why LMT chose 3D printing to reduce the weight of their lifesaving incubators and take core components into series production.
Industry
Medtech
Key solutions
- SLS
- Polyamide
- Manufacturing services
The impact
- Lightweight
- Flexible but sturdy
- Suitable for series production
- Print on demand
The challenge
Print lightweight parts that conform with medical regulations
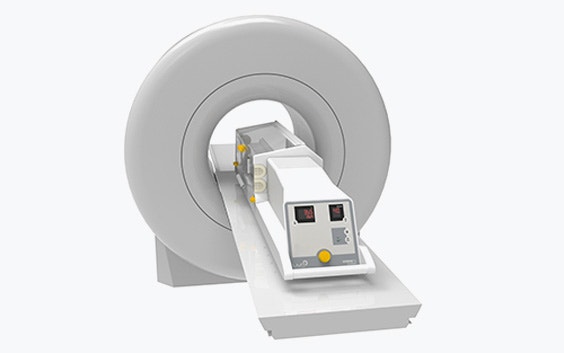
LMT Medical Systems makes incubators with a difference. Like traditional systems, they offer lifesaving environments to premature babies, but their unique design sets them apart. With an LMT incubator, medical staff can transport newborns and premature babies directly from the NICU into the MR suite for an MRI — something that was impossible before. The baby is protected throughout the MRI process, opening new doors to potentially lifesaving examinations.
“The MRI compatibility makes our system special,” explains Judith Müller, a Mechanical Engineer at LMT. “How can we do that? Because of the special materials we select and by shielding our electronics system. We also provide a power supply that runs on batteries, making it suitable for intra-hospital transport.”
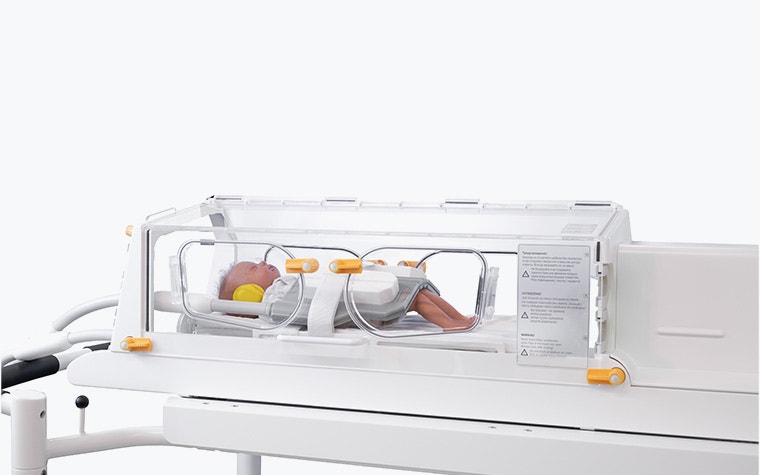

“We produce 10 to 20 systems per year for customers around the world, from China to the US and Europe as well,” says Judith. “That includes both the incubator and the RF coils. The project is individual for each customer, and we must also meet the medical device regulations.”
Understandably, LMT faces several regulatory requirements that come with creating a medical device like this. Beyond conforming with the medical device regulations, every material must have specific characteristics.
“The first thing is that they cannot be magnetic,” explains Judith. “The other is ensuring that they aren’t visible in the MRI image. We have to consider both for each material we use. Of course, we also need to meet the requirements for electrical safety standards and provide insulation for the electrical parts.”
Alongside these requirements, the small number of units produced each year effectively ruled out traditional manufacturing techniques for serial production — at least without having to store large quantities of parts.
The solution
Redesigning for AM and printing with SLS
Since 2018, LMT has produced a large number of components in polyamide through 3D printing, seeing the technology as an ideal fit. Importantly, this followed a decision to redesign their incubators for additive manufacturing — the best way to optimize what the technology offers. After designing the new system in-house, they came to Materialise to bring it to life.
“We first included 3D-printed parts in an earlier development, around 2016, when we added a new head coil,” Judith tells us. “This new design started with the idea of using 3D printing. The complete housing is made out of 3D-printed parts. There are also parts that need threads inside, so we use different techniques — for example, we use thread inserts or even include them directly in the material.”
Since then, LMT has used SLS to manufacture the incubator. By printing the parts individually, the LMT team can assemble the machines easily before sending them on to their customers. For example, SLS-printed parts are glued together and finished with a coat. Being able to print parts quickly and on-demand — an inherent benefit of series production through additive manufacturing — is key to maintaining a short lead time.
Similarly, LMT’s decision to use polyamide has continued to pay off.

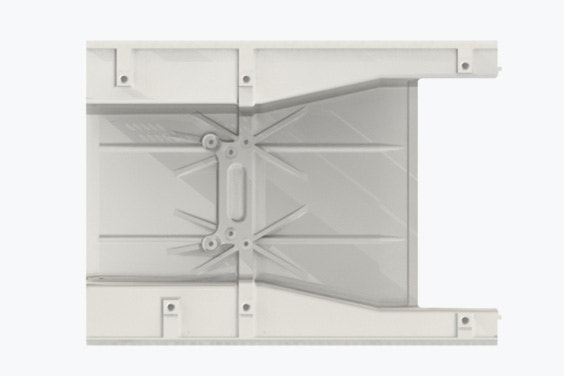
The team prints unusually large pieces, including the machine cover shown here. ©LMT Medical Systems
“What’s really good about polyamide is that it’s tactile. It’s very flexible but solid as well. We ensure biocompatibility through the coat we use, so it’s not a requirement of the material itself. However, the coat coming off is a risk point, so making it out of a safe material like PA 12 helps,” she explains.
“In the past, I’ve thought about using a different material so that we don’t have to add the additional coat. I checked other technologies like FDM, for example, but you always have visible layers on the surface, making it unsuitable for medical devices. So, I always come back to polyamide and SLS due to the cost and the possibilities that come with printing in powder, such as not needing to add supports.”
Judith’s latter point is particularly important considering LMT’s preference for series production. Each order consists of one to ten pieces of each part, removing the need for excessive storage. Relying on a digital inventory also allows the team to be flexible in optimizing the products — something Judith is keen to do alongside Materialise.
The result
Regulatory compliance and a weight saving of 15 kg
Having landed on a winning combination, the LMT team is happy with their latest design — they conduct their own income controls and any small post-processing changes on every part they receive in-house — and the results speak for themselves. Not only do the machines conform with the all-important medical regulations, but switching to 3D printing comes with additional benefits. Now, it’s all about continuous improvement.
“What’s nice about this development process is that we know we can meet all our requirements from the beginning while still having a lot of design flexibility to make complex components,” says Judith. “One of the main reasons to develop this new device was to save weight. The incubators need to be lifted, and the last model weighed around 45 kg. Now, it weighs 30 kg, and we’ve even begun hollowing out our parts to make them even lighter. We’re just getting better and better.”


An ongoing relationship is an important part of that improvement, and it’s one that Judith and the LMT team appreciate.
“Working with Materialise has been a very nice experience from the start,” she says. “After first meeting with the project engineers, we had the chance to decide whether to order online through Materialise OnSite, which we often do for small parts, or turn to them for help on the more complex, bigger parts, which we also appreciate. It's great to know that we always have the chance to speak to Materialise, get good input, and work together.”
Share on:
