EXPERT INSIGHT
How to Go Beyond Typical R&D Usage with 3D Technology
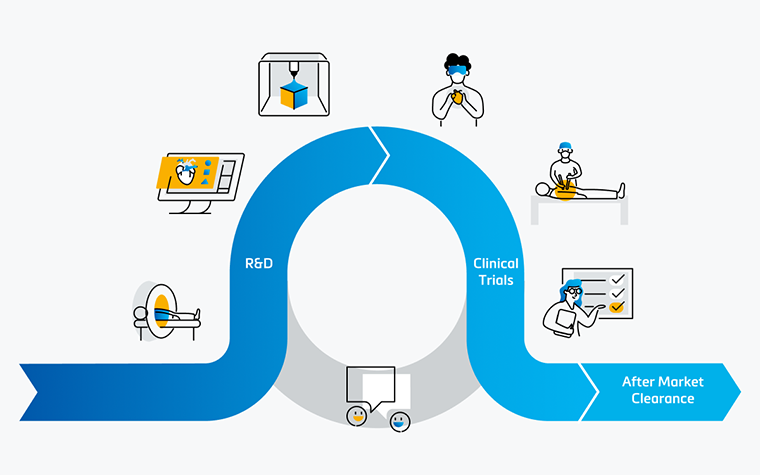
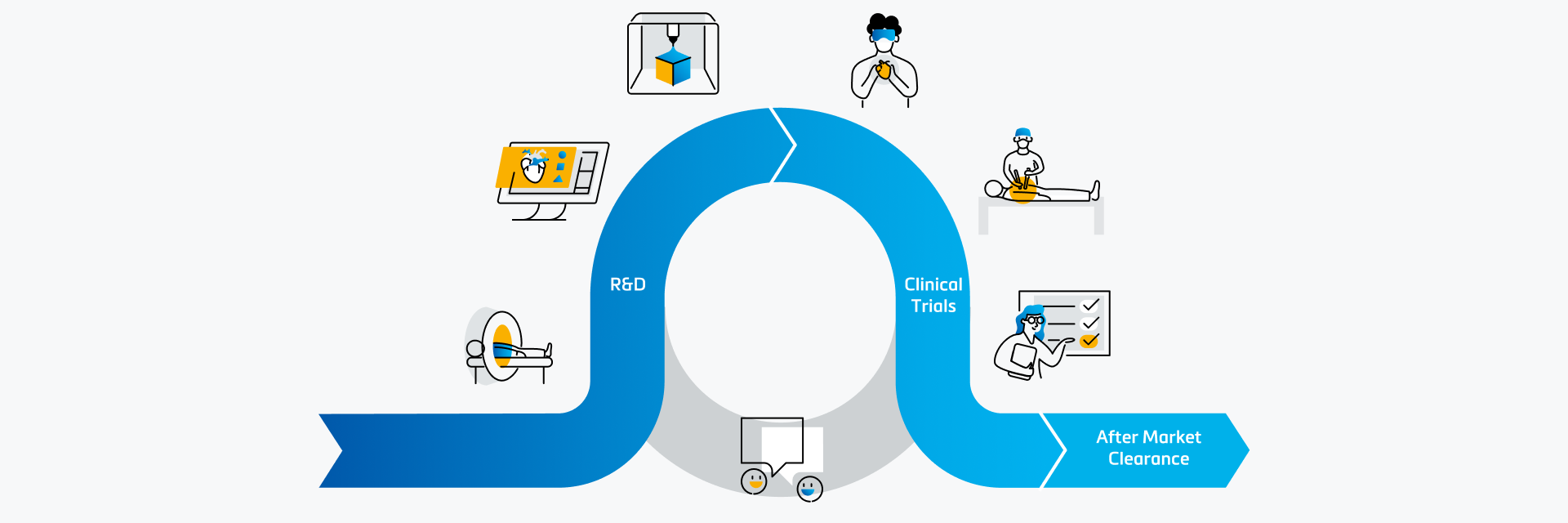
Medical device companies are facing more challenges than ever before. Not only is it a very dynamic and fast-growing market with fierce competition, but the difficult economic times also force them to reduce costs. These challenging circumstances compel companies to think outside of the box, innovate on new territories, and push themselves to explore unconventional solutions. One of these novel routes is to explore the use of 3D technology beyond the traditional research and development (R&D) usage. This article aims to give a perspective on other aspects where 3D technology can also have an impact.
Benefits of typical R&D usage
The use of 3D technology in medical device design has become an integral part of the R&D process and is seen as the golden standard by many companies. The benefits can be subdivided into two main categories.
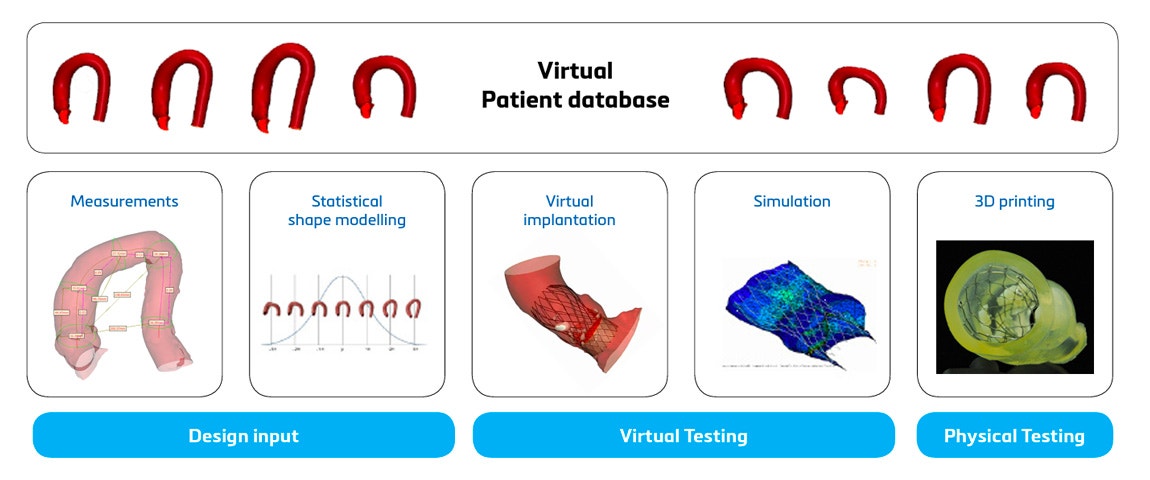
The first main usage is at the beginning of the R&D process to gather relevant design input. With 3D technology, researchers gain an accurate and complete understanding of the anatomy of the target population. This level of precision goes beyond traditional research methods, allowing for a more tailored approach to device design. A widely used method to gain these insights is by using 3D analysis. This enables the creation of a digital twin of the anatomy of interest, allowing the research team to get a three-dimensional understanding of this anatomy and collect quantitative input by performing relevant measurements. A detailed example of 3D analysis’ impact is Innoventric, a tricuspid replacement company that brought their solution to patients faster with the tech. The use of 3D technology can be taken one step further with Statistical Shape Modeling (SSM). This method allows us to not only look at the 3D data of each individual patient but also analyze patterns and average and worst-case scenarios among a target population. In fact, SSM helped Venus MedTech, China’s leading transcatheter heart valve company, make their structural heart device design more reliable.


The second significant benefit is that after a prototype has been developed, you have the ability to quickly pinpoint potential design issues through verification. Often, medical device companies opt to verify their prototype both virtually and physically. Virtual testing is also known as in silico trials and is explained in more detail in this article about using digital twins. The most cost-effective and accurate method to physically verify a prototype is performing benchtop tests on a 3D-printed model. This well-established method is explained in more detail in this article, where Medtronic describes how they use 3D-printed benchtop models for aortic aneurysm device testing.
Enabling the clinical team to excel
The impact of 3D technology can be exploited much more than the typical R&D usage. Even when the design of a new medical device passes beyond the R&D phase, the R&D team can still have a tremendous impact on the success of this device. Below, five important examples are described:
- As the device passes the verification & validation phase, the next step of human trials is often taken up by a different team. To ensure a smooth transition to this clinical team, the translation of how 3D technology can be used in these clinical trials plays a key role in its success. This article details how 4C Medical Technologies brought their mitral replacement device to the market by leveraging 3D technologies at different stages of development.
- 3D-printed models are not only beneficial in the R&D stage to perform benchtop testing but can also be used in the clinical phase for clinician training and education. Anatomical models can be used to educate internal teams, facilitate communication with physicians and patients, and train physicians when using a novel device. This article by IMMR highlights the benefits of 3D models to speed up innovation.
- As clinical cases increase, companies are forced to optimize their workflow. One way to gain consistency while reducing the time spent and the learning curve of new employees is to automate the 3D processing workflow. Here, the knowledge of the R&D team is a huge advantage as they already know all the ins and outs of the steps needed (segmentation, measurements, virtual implantation, reporting…) and how to automate this. More information on how automation can help scale up your personalized medical device business, can be found in this webinar.
- Many medical device companies still rely on the expertise of their R&D team when it comes to complex clinical cases. The complexity can be reflected in many ways: images that are difficult to import or segment, patient anatomy, additional required analysis, fluoroscopy planning to define the optimal C-arm angle in the cath lab, and more. In all these cases, the knowledge built over the years by the R&D engineers plays a significant role.
- Lastly, the 3D expertise of the R&D team is an important asset to efficiently perform post-op analysis that can be used to build evidence, convince regulatory bodies and physicians, and ultimately increase market share.
Advantages that lead to next-generation R&D
Apart from the typical R&D usage and the support in clinical trials, 3D technology also offers key advantages when developing next-generation R&D. By involving the R&D team (closely or only from the sideline) in the clinical operations, the company does not only profit from its expertise to enhance the clinical cases, the R&D engineers can also quickly pinpoint potential shortcomings of the current device, leading to opportunities to improve the next generation device.
This article describes an example of this by DJO, a key player in the orthopaedic surgical device market. While starting their clinical operations, DJO noticed some shortcomings in their current hip implant, which they optimized thanks to image-based population analysis, leading to a successful relaunch of their next-generation hip implant.
Wrap-up
The continuous loop between R&D and clinical operations is one that can only be strengthened with the usage of 3D technology. It offers widespread benefits along the product life cycle for medical device development. From gathering design input and prototype verification in the typical R&D usage, the support of the clinical team, to next generation R&D; 3D technology is reshaping the landscape of medical device design.
L-103749-01
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.