CUSTOMER STORY
How Statistical Shape Modeling Supports Structural Heart Device Design with Reliable Analysis
What does China’s leading transcatheter heart valve company need in their toolbox to stay at the top despite stiff competition? After speaking to Venus MedTech, we learn that it is a twofold answer. They need the correct tools for R&D to meet their goal of solving unmet needs in the structural heart space. Better yet, if the tool covers population-based analyses, they'll benefit from improved credibility with regulatory bodies when they bring it to market. With 3D technology enabled by Mimics, they can check both requirements off their list. They can gain accurate patient data faster while improving their case for the market. Through this powerful collaboration, they expect to continue leading the Chinese market in lifesaving structural heart devices.


Start from a medical need
Founded in 2009, Venus MedTech was the first to bring a transcatheter aortic heart valve to market in China. And recently, they CE marked their pulmonary valve, so they are used to being ahead of the curve as innovators. Now, with over 800 people on their team, they aim to become a global company and are working on replacements for all four valves.
To start working on a new valve, they begin from a medical need and really lean into clinical feedback. The accuracy provided by Mimics creates a reliable basis for measurements and is a benefit that helps them precisely convert clinical needs into innovative devices. Lim Hou-sen, Venus MedTech's CTO and COO, explains why 3D data is so essential for them and adds, "As device manufacturers, the understanding of the anatomy for the application of treatment is always the basis. For valves, it is geometric. The other challenge is dealing with biology and diverse shapes and sizes. It's not only about the variability but a different combination of key dimensions and the relations between them. That's why it is so important that we have a good understanding of spatial requirements when developing a device."
Tedious manual labor before 3D
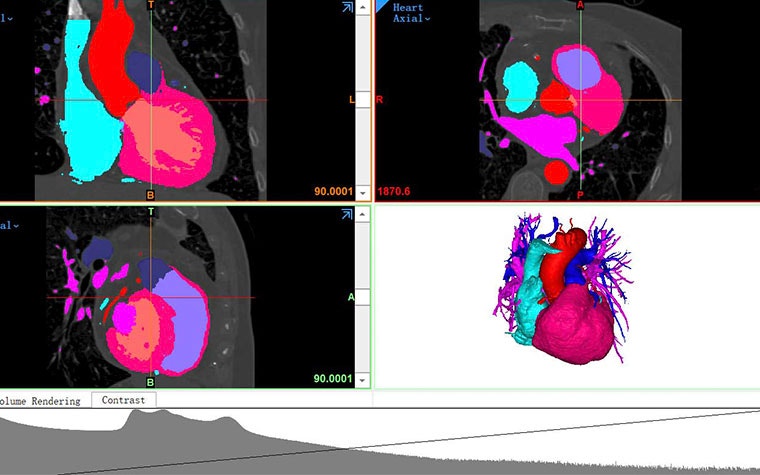
Before Venus MedTech used 3D technology, they tried another system, but there were a lot of manual steps going from receiving physician input, performing measurements, tabulating spreadsheets, and more. Now, instead of skimming through hundreds of CT scans to figure out a representative shape and working through a lot of trial and error, they can use 3D modeling in Mimics to automatically segment and visualize the anatomy in 3D. This gives them an accurate basis for measurements before going on to use the Statistical Shape Modeling (SSM) module. SSM is a much faster way in which geometric models describe a collection of similar objects in a very compact form. SSMs can represent an average shape of many three-dimensional objects and their variation in shape, which supports their work by gathering an average of different shapes instead of just using a few use cases to reference different anatomies. Mr. Lim adds, “The big benefit that SSM brings is the visualization. We can easily make pre-physical models to test our devices from the model created.”

“The most important benefit from SSM is that the results of the anatomy we choose come with strong statistical meaning.”
— Sihang Cheng, Venus MedTech R&D Manager
Mapping out worst-case scenarios
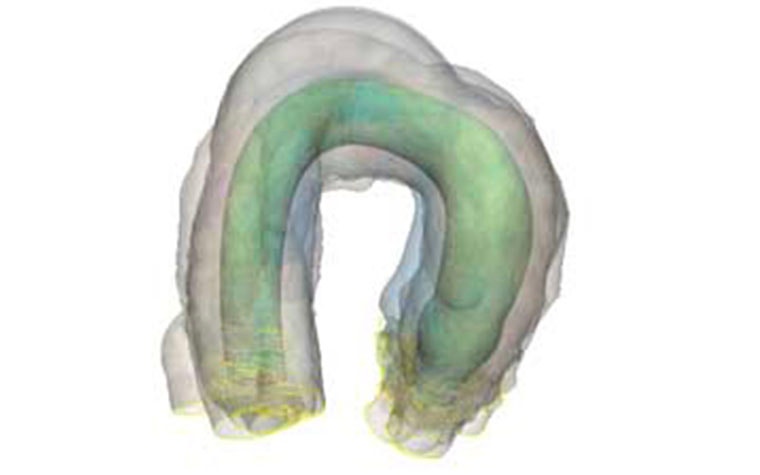
With the benefits of using SSM, Sihang Cheng, Venus MedTech's R&D Manager, hopes they can use it on their newest challenge — creating a new generation valve for aortic regurgitation patients. Aortic valve calcification is very severe in the Chinese population, so it's exceedingly difficult to plan for the delivery system in the left ventricle. That's why they use SSM to find the worst-case scenario to see if their delivery system works. Mr. Cheng adds, "It depends on the R&D stage, but we would like to use the most average anatomy to try our design and delivery system at a very early stage. But when we go through the validation and verification key phase, we like to try something extreme to see what we should avoid. This is a way of knowing if there is anything we can do to improve our device."
SSM will also help inform them on what sizes to make and what range to fit most of the population that would use the device. Their goal is to aim for 80-85% of the population. With this module in Mimics, they have been able to increase efficiency, which in turn, reduced the R&D team's workload.

Strong statistics for regulatory
Another benefit is that they can create true physical models from accurate data. Mr. Lim explains, "The more patients of your target population that you can include, the more statistically relevant your data becomes, and that's what really helps in convincing regulatory bodies." It all goes back to the data as Mr. Cheng adds, "The greatest benefit from SSM is that the results of the anatomy we choose come with strong statistical meaning."
In the future, Venus MedTech will continue with segmentation as the basis of their work and use SSM across departments. The data they have gotten has given them more precise and reliable anatomical numbers so they can go to regulatory bodies like the FDA with more confidence. They are also working on new generations of devices and already have a new iteration of their Venus A valve. Mr. Lim explains, "For every generation, we want to improve or widen the scope of use or improve the usability, which is why it is important to find the limits." They are looking forward to seeing additional AI algorithms in Mimics and Mimics Viewer to help further automate their work and predict even more outliers.
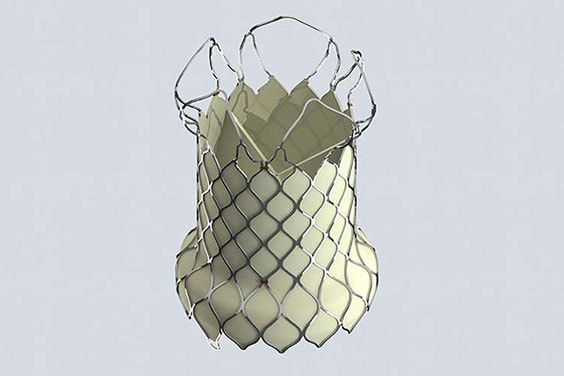
VenusA-Valve, the first transcatheter aortic valve replacement system approved in China, and VenusP- Valve Systems, the first self-expanding pulmonary valve in Europe known to Venus MedTech.
Ensuring positive future outcomes
As for advice for other medical device companies that want to design a device that fits a specific population, Venus MedTech believes their formula isn't unique. Mr. Lim advises, "Work with the best doctors, with an open mind and that are willing to share their experience. Work with the best engineers and have good tools in this process — we are glad to know Materialise as a company that listens to feedback and continuously improves software and our tools. I know if you have a good team and good clinical input together, you always get a good outcome." We look forward to continuing our collaboration and seeing how Venus MedTech progresses in their goal of solving unmet clinical needs in the structural heart space while focusing on innovative ways to ensure patient safety.
L-102742-01
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.
