CUSTOMER STORY
4C Medical Technologies Works with Materialise to Bring Mitral Regurgitation Solution to Market
For medical device start-ups, speed, efficiency, and money are particularly crucial when bringing a solution to market. Dr. Saravana Kumar of 4C Medical Technologies shares insights into why the right tools and the right partner are key when going from idea to patient care.
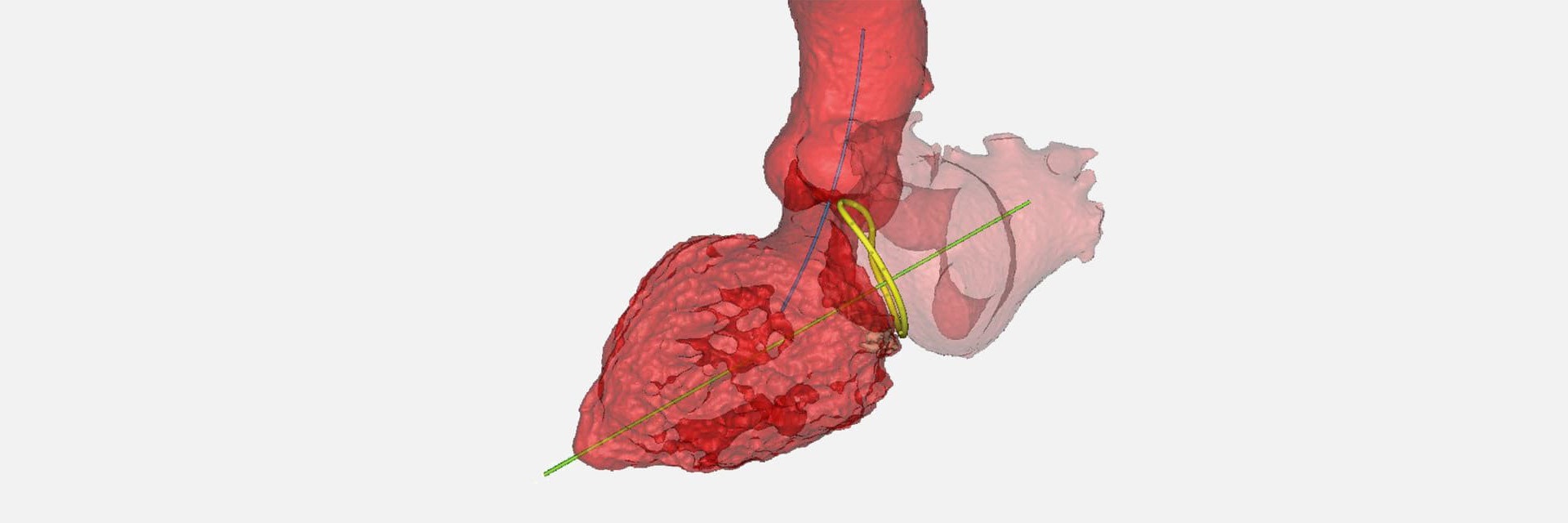
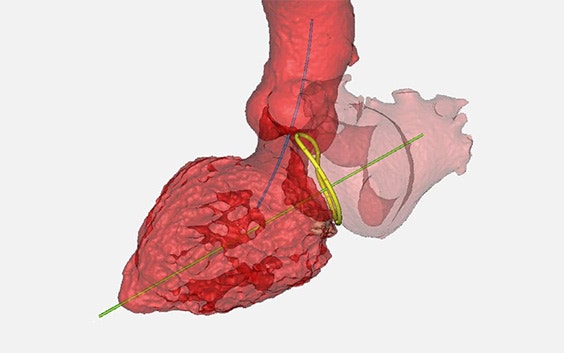
4C Medical Technologies is a medical device company working on minimally invasive solutions for structural heart diseases. Vice President of R&D and Operations Dr. Saravana Kumar and his team were working to bring their award-winning AltaValve to market, an innovative solution to addressing mitral valve regurgitation (MR). MR is one of the most prevalent heart valve diseases, where the mitral valve's leaflets fail to close completely, allowing blood to flow backward from the left ventricle into the left atrium. Thanks to two delivery options, the AltaValve device is easy to use and suitable for any anatomy in the patient population.
“We need to be efficient in what we do. This is where the relationship with Materialise and using the right tools to help us succeed play a huge role ”
— Dr. Saravana Kumar, Vice President of R&D and Operations, 4C Medical Technologies
Starting as a startup
As a medical device startup, there are key elements that need to be met in order to stay afloat and eventually make it in time to market before funds run out. Dr. Kumar shares necessary elements for a successful and efficient process from idea to patient care, in one of our webinars (23 minutes): a great, simple idea that addresses a specific need, people with the right skillset to make it happen, the right technology. and of course, luck. Dr. Kumar also emphasizes the importance of speed, efficiency, and money.


"Time is of critical importance for a small company. We need to be efficient in what we do, and we need to do it in a timely manner because time equals money. There is a limited amount of funds, and it is up to us to show that our technology works and that it works consistently for us to be successful. This is where the relationship with Materialise and using the right tools to help us succeed play a huge role,” says Dr. Kumar
From innovation to implementation
Dr. Kumar further elaborates on the multiple activities in which his team works with Materialise and Mimics to maintain a fast pace. He separates these into four main phases:
- Product development: Fully understanding the cardiac anatomy, in terms of numbers, distention, and movement. And using 3D models to further understand how the anatomy interacts with the device.
- Liaising with regulatory bodies: Convincing regulatory bodies of the safety performance of the device. Understanding patients' anatomical boundaries and assessing them through realistic tests.
- Clinical phase: Creating and maintaining an efficient interaction between engineer and physician. Gathering data on post-up results is essential.
- Educational/training applications: Developing tools to train physicians in using their device. Increasing confidence in clinicians for the use of the device is equally critical to success.
Product development with Materialise
Dr. Kumar continues his presentation with a more in-depth explanation of how his team uses Materialise to understand the size and shape of the device, determining the number of sizes, depending on the target population, as well as understanding anatomical dynamics.
He also shows a realistic training model of cardiac anatomy that his team has been working on, using Materialise software. An important point in developing the model, he says, is the efficient collaboration with physicians and their feedback on the cardiac model, and how this helps them refine their device.
These are just a few examples Dr. Kumar selected to demonstrate their collaboration with Materialise and their plans for the near future. But it doesn’t end there.
Working with patients
At the time of writing, 4C had already made its device available for compassionate use to a group of patients. Results had been very encouraging and were paving the way for the device’s success as an innovative technology, improving the lives of many patients.
L-102646-01
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.