CASE STUDY
Tricuspid Valve Regurgitation: 3D Technology Accelerates Life-Saving Ideas to Reach Patients Faster
Previously considered the ‘forgotten valve’ due to the focus on the mitral and aortic valves, the tricuspid valve is finally in focus. With the help of 3D technology, Innoventric brought their forward-thinking solution to treat tricuspid regurgitation (TR) from idea to realization more quickly.
Industry
Healthcase
Key solutions
Materialise Mimics
The impact
Powerful 3D visualization
Data-driven device design
Virtual implantations
Effective patient screening
Challenge
Accelerate the R&D phase of a novel device to treat TR
When patients suffer from tricuspid regurgitation (TR), it increases vein pressure, and they can develop edema, leading to eventual multi-organ failure. The current treatment involves diuretic drugs to promote fluid loss and going to the hospital when it gets worse. The problem is that tricuspid valve conditions are mechanical problems that cannot be completely treated with just medication and will eventually require surgery to reduce symptoms and the risk of complications.
However, open heart surgery to replace a tricuspid valve is high risk for older patients. Replacing the tricuspid valve is much more complex than transcatheter aortic valve implantation (TAVI), the procedure for improving a damaged aortic valve.
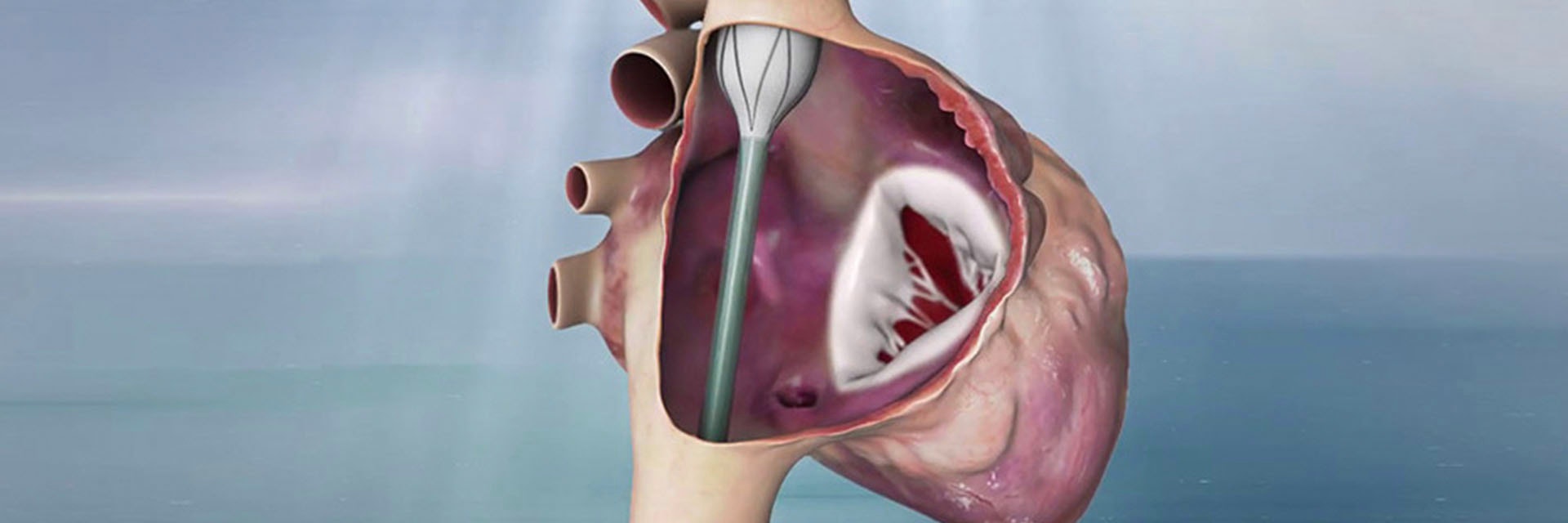
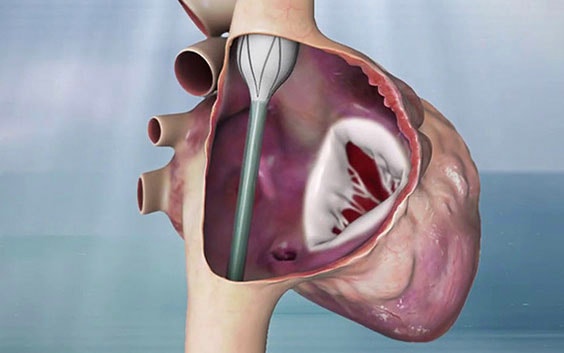
The tricuspid valve is not circular, and the annulus’ 3D shape differs for each patient — making it difficult to anchor. Additionally, the shape changes during the cardiac cycle along with chordae tendineae and papillary muscles which is not the case for the aortic valve.
Amir Danino, CEO and Founder of Innoventric, developed the idea of not replacing the valve in the native place due to the complexity of the location but adding another valve that prevents backflow.
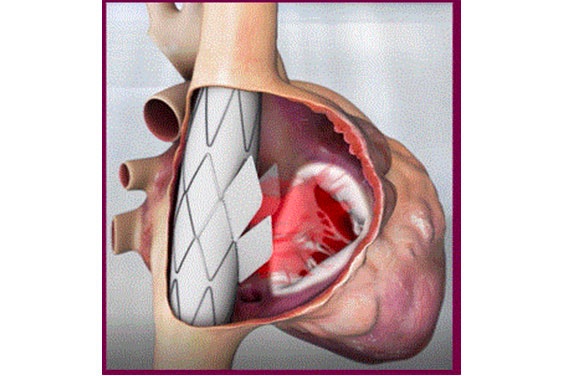

This meant not working on the tricuspid valve itself. They put a stent graft in the superior vena cava (SVC) to the inferior vena cava (IVC) with a valve in its sidewall. The anchoring is much easier due to the tubular structure and small size (in comparison to the TV annulus) of the cava veins. Rotational and longitudinal markers ensure that the valve is placed in the correct position and direction.
To design a valve with the correct sizing, the team needed some crucial measurements such as the diameter of the IVC/SVC, stent length, inlet to the right atrium (RA), distance from the septum, RA wall distance, and RA volume.
“When it’s 3D, it’s easier to understand the anatomy and challenges that you will have to deal with during the procedures.”
— Yair Pichersky, R&D Project Manager at Innoventric
The solution
Materialise Medical and Mimics
Innoventric reached out to the Materialise Medical team, who did an analysis including segmenting the datasets and performing some measurements.
With the virtual model and the measurements, Innoventric was able to interpret the results and use them for validation and verification (V&V) by defining if the diameter, length, and shape of the stent graft worked or should be adapted. They then were able to define if the idea of the stent graft could work — if it could be effectively delivered, and if it deployed properly in place.


Before and during the pre-clinical trial, they also examined their implant design in a pulse duplicator system that they built to be able to test it in a hemodynamic environment. As part of the V&V plan before the first in-human (FIH) clinical trial, they performed all the standard relevant tests — durability, fatigue, biocompatibility, sterilization validation, and others.
For the animal tests, they implanted the Trillium™ in 30 pigs with acutely created TR. CT scans or 3D prints were not used in the animal trial plan because the pigs had no abnormalities or variations in the right side of the heart, so sizing was standard.
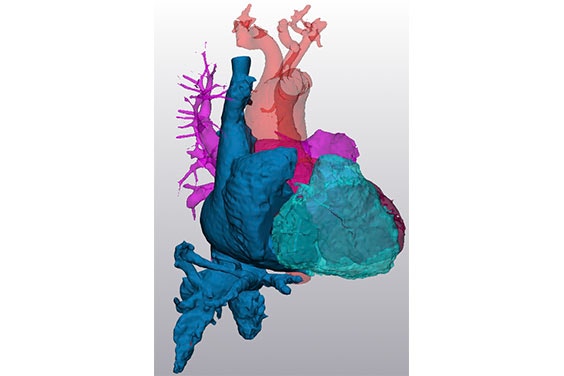

In early 2020, the Innoventric team began their first human trials. When it came to screening, the team chose patients by screening for a variety of medical variables.
They used a color Doppler ultrasound that shows the direction and pressure of the flow to determine the grade of the regurgitation. Then a CT to take measurements including the diameter at different locations of the IVC/SVC and the distances between the close hepatic veins to the right atrium to define if the device would fit and seal. In addition to anatomical measurements performed with Mimics, they also took hemodynamic conditions — pressure measurements from the right atrium, right ventricle, pulmonary artery, and the cava veins.
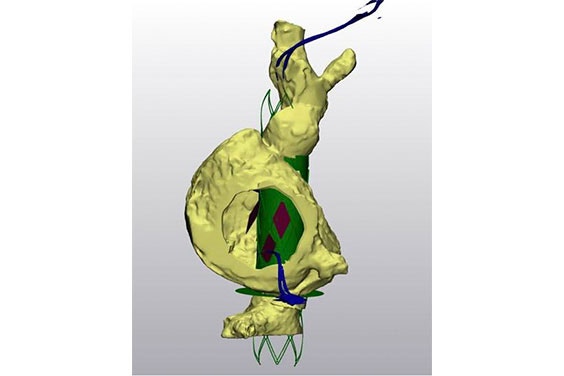

If the patient passed the screening, the team then planned the procedure using segmentation and a model. With the patient’s measurements, they were able to test out a representation of their device in 3D using virtual implantation. Afterward, they would send the data to the physician to receive feedback on the results. They also printed out pliable models and used them to train for the procedure.
Their method was to make a model, practice it in Israel, and then train the operators on-site. Using fluoroscopic markers, they can see and place the device in the correct position and direction of flow. With this workflow, they have successfully helped multiple patients and will continue with more for the FIH trial completion. As Yair Pichersky, the R&D Project Manager at Innoventric, explains, “When it’s 3D, it’s easier to understand the anatomy and challenges that you will have to deal with during the procedures.”
The result
An efficient journey through R&D to V&V and onwards
In the future, Innoventric will likely preserve their workflow as it has been successful so far. Additionally, Mimics has been a powerful tool in assisting with screening and successful simulation of device implantation. Their goal of the initial patients for FIH and beyond when they scale up is within reach for this innovative company with the right 3D toolbox by their side. Yair adds, “We are coming to the procedure with much more data and knowledge, and this knowledge gives the operators confidence as well.”
The benefits of 3D technology have been integral for Innoventric to go from R&D to validation and verification and will remain essential throughout the process all the way to clearance. Their partnership with Materialise Medical has enabled them to take the idea for the device from ‘bench to bedside’ — directly to the patients who need this innovative device.
L-102225-01
Share on: