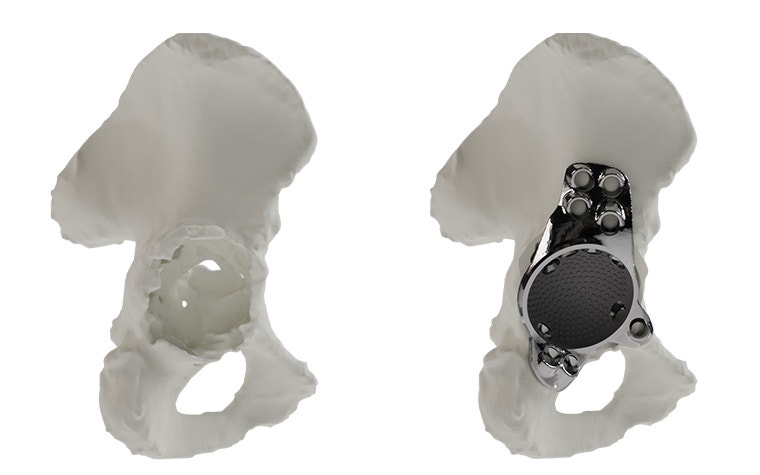
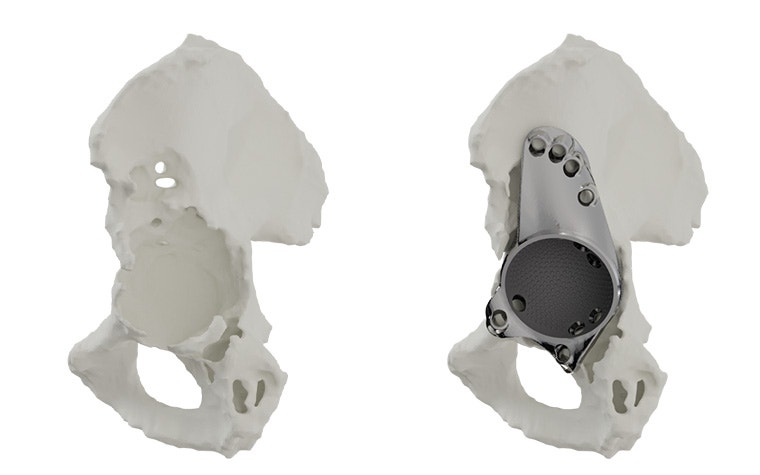
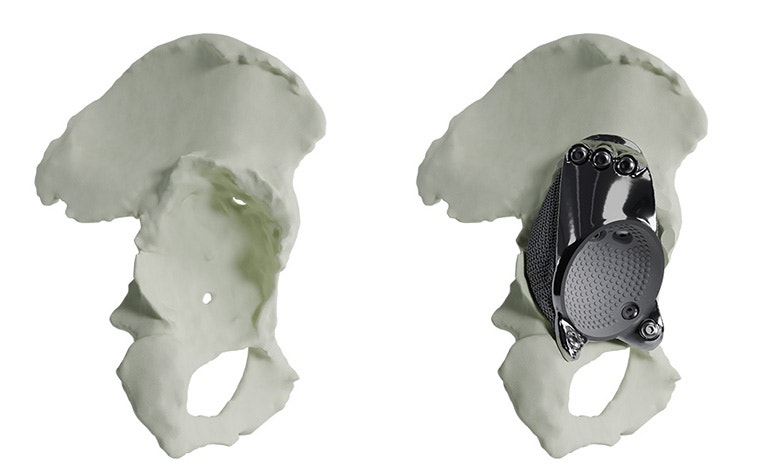
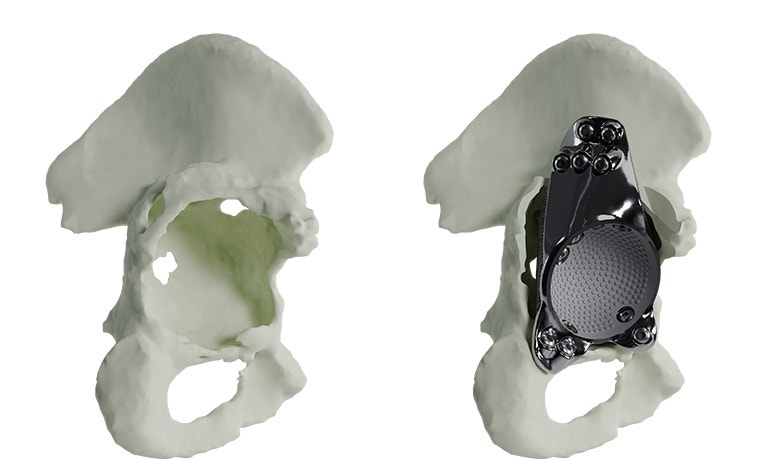
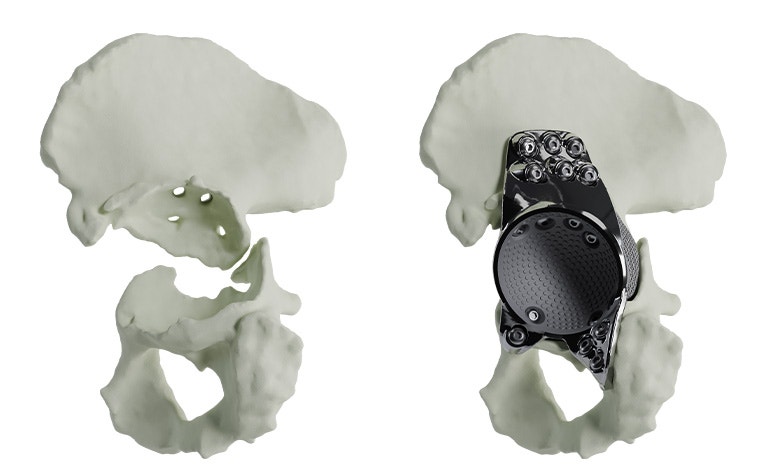
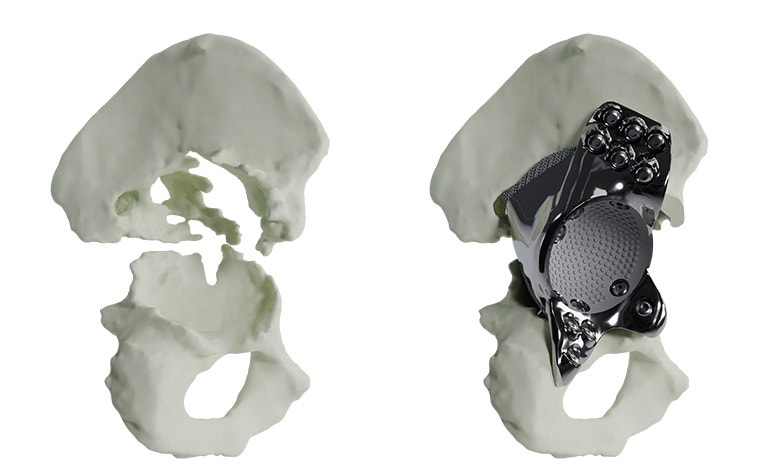
Materialise aMace
Personalized acetabular implants
Up to 27% of revision total hip replacements are re-revisions due to a suboptimal fixation and biomechanical reconstruction1. And unfortunately, re-revisions are three times more likely to fail compared to the first revision2. The aMace personalized implant is designed to break this revision cycle.
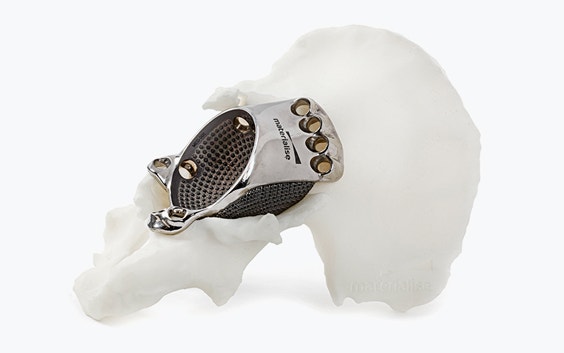
Why choose aMace for hip revisions?
Rely on a 98% patient satisfaction rate3
Patients see substantial improvements in mobility, daily activity, and pain relief.4
Trust in a 97% implant survival rate5
Follow-up studies show radiological loosening in only 2.8% of cases — lower than the reported 4% for other custom triflange acetabular cups in severe defect cases6.
Based on 15+ years of experience
Our clinical engineers are uniquely positioned to help you plan a personalized treatment.
Over 2,000 complex cases made easy
The majority of queried surgeons stated that the aMace solution brought ease to even the most complex hip revision surgeries.7
How you can break the hip revision cycle
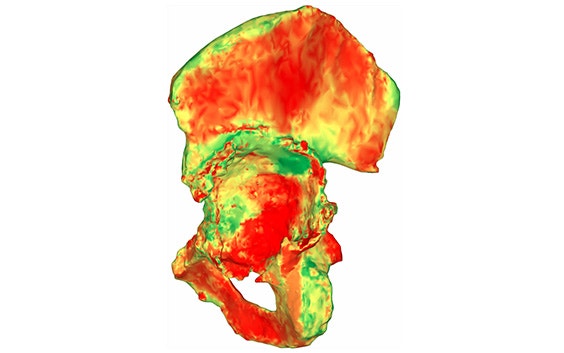
Unique 3D pelvis analyses
Based on the segmented CT scan, our experienced clinical engineers implement AI-enabled technology to quantify the acetabular bone loss and available bone stock by measuring the thickness of the bone and cortex over the entire surface.
Minimize risk of dislocation
Our clinical engineers minimize the risk of dislocation by preoperatively planning the position of the center of rotation as well as inclination and anteversion angles.
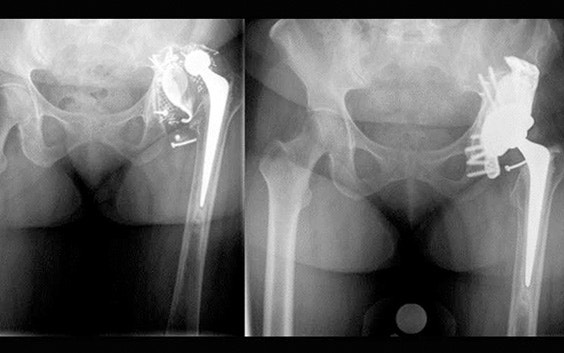
Optimized long-term fixation and stability
aMace implants are designed to optimize fixation with crossed screw trajectories. The integrated augment mimics bone properties and is designed to improve secondary fixation through bone ongrowth.

Patented drill guides
Innovative 3D-printed guides allow you to optimize primary fixation by placing the required cross-fixated screws as indicated by the detailed preoperative plan.

MDR compliant
Materialise aMace is considered a class III custom-made implantable device under MDR and is covered by QMS certificate G13 110051 0001.
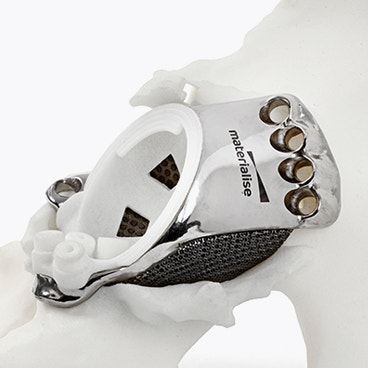
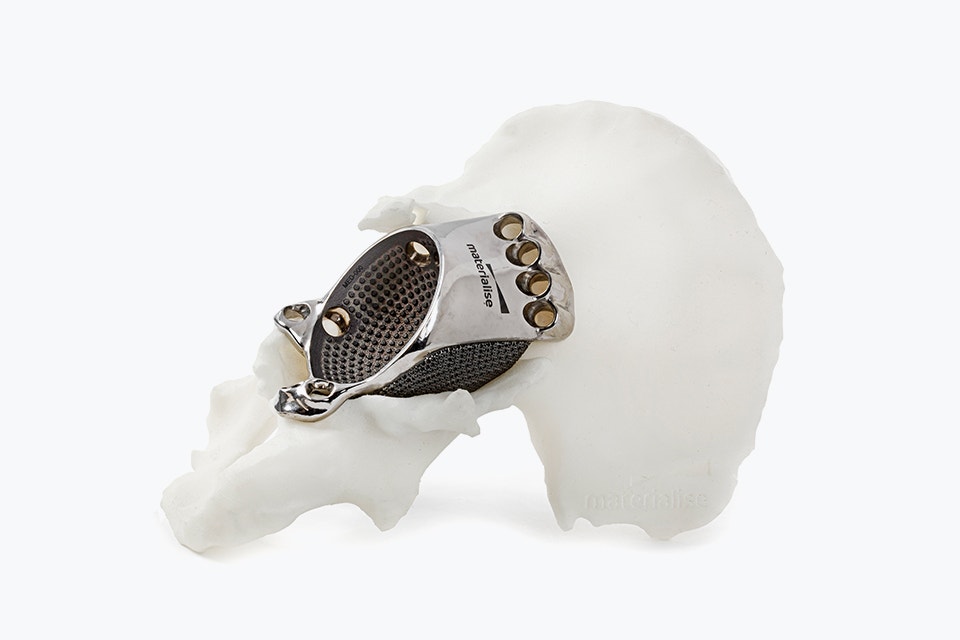
The features of an aMace implant
The aMace personalized acetabular implant is a one-piece solution, made of titanium alloy (Ti-6Al-4V) — a material preferred for orthopaedic applications due to its biocompatibility, mechanical strength, corrosion resistance, and osteointegration.


1) Porous structure:
The material properties mimic the mechanical properties of trabecular bone. Secondary fixation is improved through a design that allows bone ongrowth.8 For large, defect-filling implants, porous structures also allow a total weight reduction of the implant.
2) Outer surface options:
Now, you can choose which sections on the implant's defect-filling structure are porous and which are polished, based on the patient’s anatomy. A polished surface can be applied in regions with complete bone loss to avoid soft tissue irritations.
3) Inner surface:
Integrated studs on the implant's inner surface enhance the adhesion of the cemented cup.
4) Flanges:
Three flanges secure the implant to the bone, with screws strategically oriented to optimize fixation in areas of highest bone quality, ensuring a stable and well-supported construct.
Indications
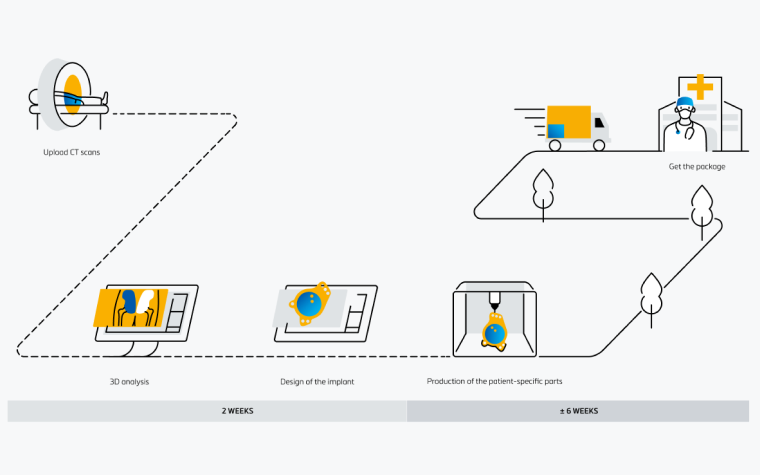
Ordering process
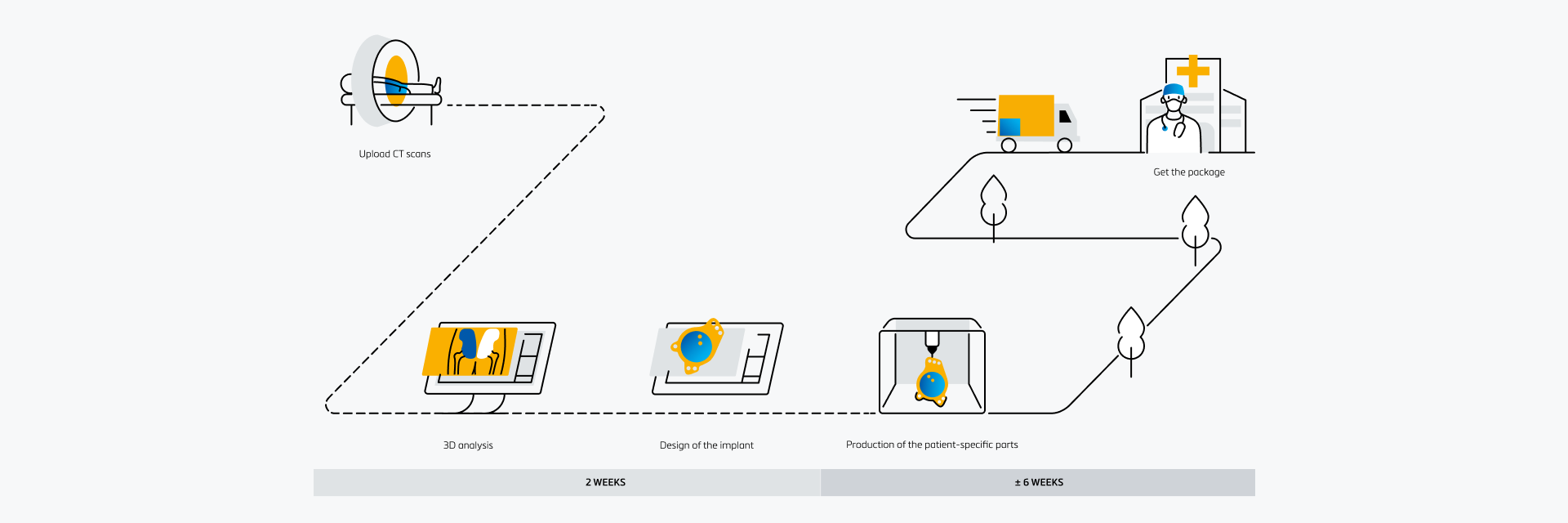
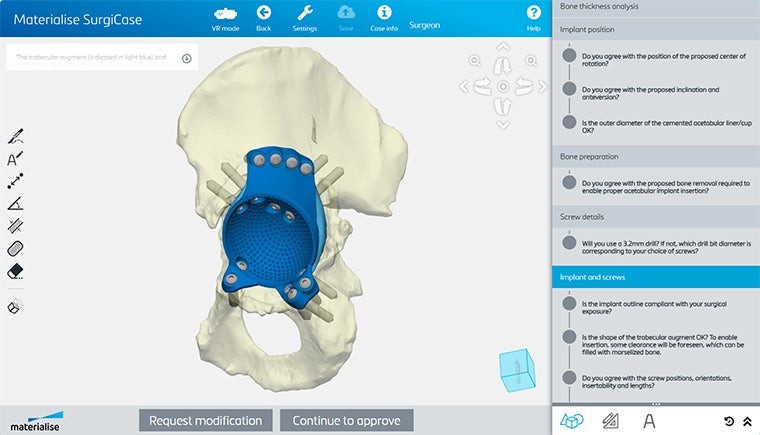
An easy-to-use digital platform
Collaborate with your team and our engineers on patient-specific solutions with Materialise SurgiCase. In this all-in-one platform, you can upload images, plan your case, and work alongside clinical engineers. Securely access the platform from any internet browser.
Clinical evidence
Access the latest articles supporting clinical evidence for the aMace hip implant. For a complete overview, please refer to the clinical data report.
Using custom-made 3D-printed titanium implants for Paprosky III defects show good agreement at the 1-year follow-up
Custom-made 3D-printed cup-cage implants for complex acetabular revisions: evaluation of pre-planned versus achieved positioning and 1-year migration data in 10 patients (2021)
Good improvement in patient-reported daily functioning, high patient-reported satisfaction, few complications, and no re-revisions for custom triflange acetabular components
Good results at 2-year follow-up of a custom-made triflange acetabular component for large acetabular defects and pelvic discontinuity: a prospective case series of 50 hips (2021)
Using a custom-made triflange acetabular cup in patients with acetabular loosening and pelvic discontinuity can result in stable constructs and significant improvement in functioning and health-related quality of life at two years' follow-up
Custom-made acetabular revision arthroplasty for pelvic discontinuity: Can we handle the challenge?: a prospective cohort study (2023)
Patients with Paprosky type 3 defects who received aMace implants demonstrated improvements in the Oxford hip score and Harris hip score from 19.8 and 50.1 to 29.4 and 68.8, respectively.
Functional and radiological outcomes after treatment with custom-made acetabular components in patients with Paprosky type 3 acetabular defects: short-term results (2020)
Get inspired
Discover how others benefit from the personalized aMace implant
Learn about aMace and get support
Explore our resources to discover more about aMace and its impact.
Frequently asked questions
L-102611-02
References:
1 Swedish Arthroplasty Register, Annual Report 2014
2 NJR 21st Annual Report 2024
3Baauw et al. 2017; Colen et al. 2013; Myncke et al. 2017; Scharf-Baauw et al. 2021
4Baauw et al. 2017; Citak et al. 2017; Scharf-Baauw et al. 2021; Gruber et al. 2020; Faraj et al. 2023
5Baauw et al. 2020, 2017, 2015; Citak et al, 2017; Colen et al. 2013; Goriainov et al. 2018; Myncke et al. 2017; Van Eemeren et al. 2020; Matar et al. 2020; Gruber et al. 2020; Scharff-Baauw et al. 2021; Augustyn et al. 2022; Faraj et al. 2023; Madanipour et al. 2022
6Baauw et al. 2020, 2017, 2015; Citak et al, 2017; Colen et al. 2013; Goriainov et al. 2018; Myncke et al. 2017; Faraj et al. 2023; Madanipour et al. 2022; Demol et al. 2012
7Myncke et al. 2017
8Demol et al. 2012