EXPERT INSIGHT
From Bridge to Series: Where 3D Printing Adds Value in Medtech Manufacturing
The medtech industry is increasingly turning to 3D printing as a trusted manufacturing tool. It’s an ideal solution for series production, especially when manufacturers identify the right applications and design with the technology in mind from the very start.
Whether driven by economic, design, or regulatory considerations, many medtech manufacturers are investing in 3D printing for niche products and functional parts throughout the development cycle.
The technology adds proven value far beyond the prototyping phase. Some companies trust it as a tool for bridge production, demonstrating the product’s performance for an extended period before switching to an alternative method as demand grows. Others rely on its design freedom, flexibility, and efficiency from ideation to series production.
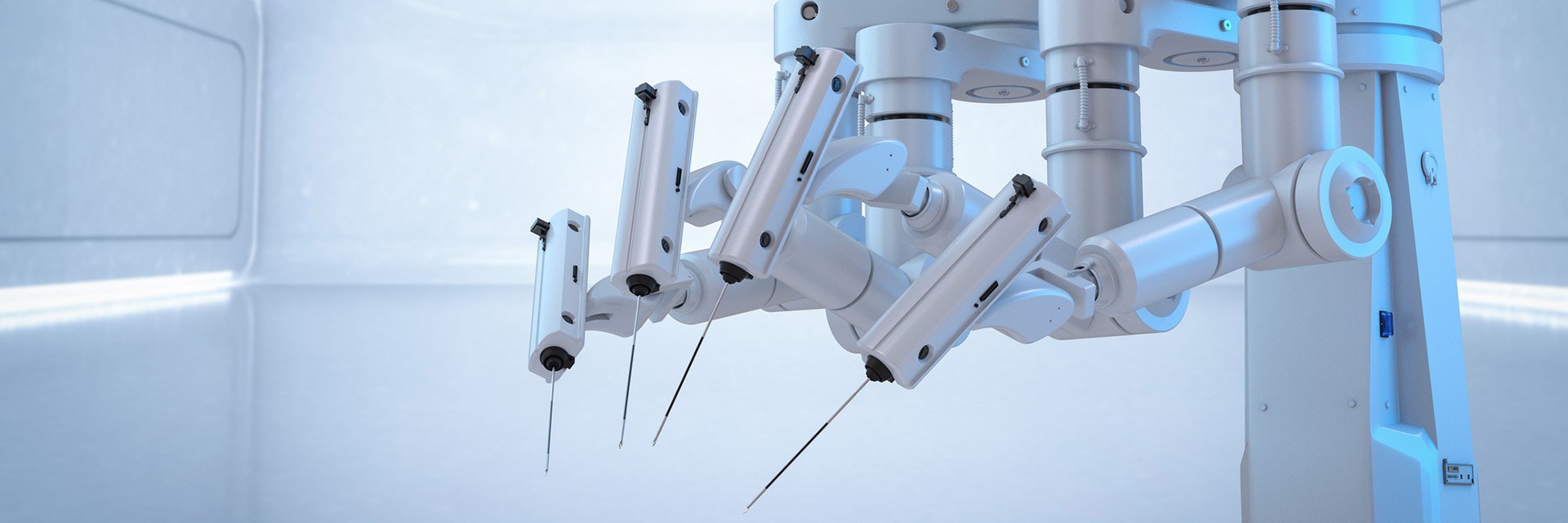
We see this trend in diverse segments across the industry, from diagnostic equipment to surgical tools. That’s because (with the right application) 3D printing solves many of medtech’s common challenges, particularly when it comes to supply chain agility and sourcing custom or low-quantity components that are too expensive for traditional techniques — think orders of 10s, 100s, or low 1,000s.
Economic and technical advantages
So, what makes an application ‘right’ for 3D printing?
Traditionally, technical drivers have been the most well-known reason for adopting the technology. The success of Ossila’s USB spectrometer, for example, was driven by design freedom, combining several components into one to streamline assembly. In this case, the team halved the number of individual components, ensured incredible accuracy, and made assembly five times faster — valuable gains for any manufacturer.
In recent years, however, what manufacturers seem to value even more is 3D printing’s economic viability when sourcing niche parts. With no minimum order quantities, it’s an ideal method for small series production. This is especially valuable in sectors like biotech and life sciences, where the production of high-value, customized machinery is more common.
Scaling to series production in biotech and life sciences
Sartorius, for example, requires biocompatible components for the 100 or so bioreactors it produces and sells each year. As many of these machines are customized to fit the needs of each end-user, the team regularly makes small changes to individual parts. This would not be financially realistic with traditional methods due to the cost of tooling. With 3D printing, any changes are made to a CAD file instead of a physical mold, making customization easy, efficient, and affordable.


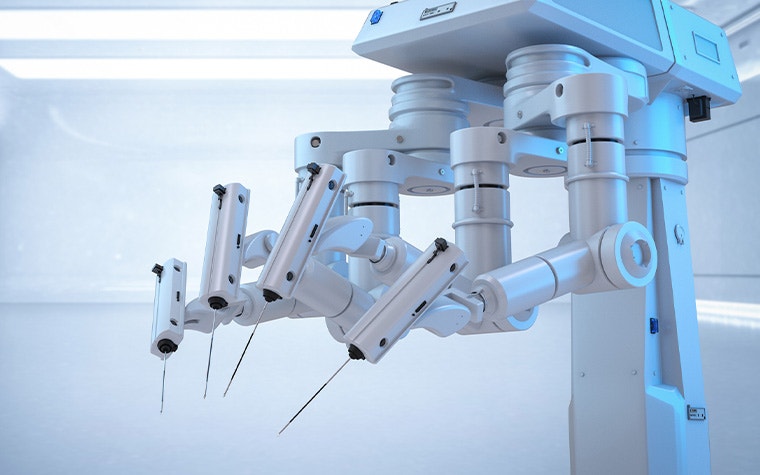
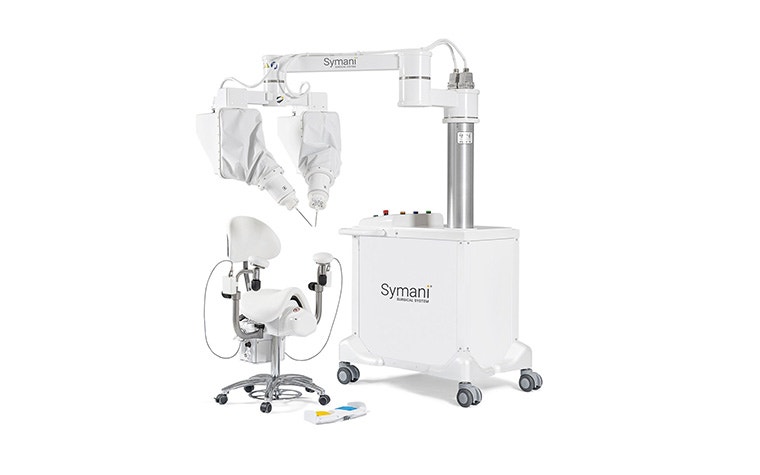
Surgical robots, such as those produced by MMI, are a similar example; a standardized solution with ever-changing parts. MMI uses 3D printing to manufacture end-use parts for their surgical kits, particularly disposable instruments and connections for the arm itself. Again, 3D printing’s suitability for complex design is a big advantage. The team designed these parts with the technology in mind, incorporating interior channels and merging multiple components to improve performance. Overall, it's a decision that helped them grow from a start-up in 2015 to launching a CE-marked product on the market in 2019.
“Working with Materialise to leverage the benefits of additive manufacturing (AM) has allowed us to really fast track design iterations on a number of components critical to the development of our microsurgery system, helping us move from prototyping to product in a much shorter time,” says Massimiliano Simi, Founder and Vice President of R&D at MMI.

“For a project like ours, this is hugely important as we need the adaptability to keep developing. With micro-instrumentation, an adjustment of just a 100th of a millimeter can have a significant impact on performance. Where switching molds for each new iteration would become incredibly costly, 3D printing allows us to be nimble with our design iterations.”
3D printing also adds value to applications in the broader category of hospital equipment, such as housing or cases for patient monitoring systems like LMT’s MRI-compatible incubators. In this case, LMT reduced weight by 33%, improving usability; similar applications may require characteristics like cleanability and sterilability, both of which are possible with 3D printing’s materials and finishes.

Getting the best results
It’s important to note what these examples share: each company decided to use 3D printing at the beginning of the project, designing their parts specifically for their chosen technology. It’s key to getting the best results.
While it’s not uncommon for companies to turn to 3D printing with an existing design, doing so can impact the outcome. Once a product is validated and released on the market, redesigns are typically out of the question, limiting the opportunity to take full advantage of 3D printing's design freedom.
“In our experience, the most successful applications happen when additive manufacturing is considered from the beginning of the product development cycle, from prototyping onwards,” explains Radhika Dhuru, Market Manager for medtech at Materialise. “A common example is products with multiple components that don’t need to be separate — with 3D printing, you can remove the need for assembly by producing it in one piece.”
Overcoming adoption hurdles with the right partner
In a demanding, highly regulated industry like medtech, manufacturers can find it challenging to adopt newer technologies. Evidence, quality, and regulatory know-how are essential — as is guidance in making the right decisions.
That’s where Materialise adds value as a manufacturing partner; we combine over 35 years of additive manufacturing experience with a deep understanding of the medtech industry, helping countless manufacturers adopt 3D printing successfully.
Crucially, we do so by working with them through every stage of the development process under ISO 9001 and ISO 13485, from pre-clinical validation to bridge or series production. Our extensive production facilities, including an ISO-7 clean room, ensure scalability and flexibility, while our experience in quality assurance, documentation, and design validation guarantees compliance with industry standards.
End-to-end support
Our design and engineering expertise is particularly valuable, especially for innovative manufacturers like MMI. As their surgical robots continue to evolve, so too does the modular surgical training board they developed with Materialise. Every time the team produces a new surgical tool, they can quickly create a new module for the board alongside it, enhancing their offer through aftermarket support. In this case, an additional segment would allow surgeons to practice three types of anastomosis between two vessels.


The development cycle for this latest addition lasted approximately ten weeks. It began with the exploration phase, where creativity took center stage. Our multidisciplinary design team translated their expertise from different sectors into innovative, feasible solutions that would not be possible with traditional manufacturing methods.
These ideas were narrowed down through the concept development and proof of concept phases, where the results of technical feasibility studies, market analysis, and cost estimates identified the most promising concepts. At this point, we also produced functional models to help refine the design before moving into the final stages.
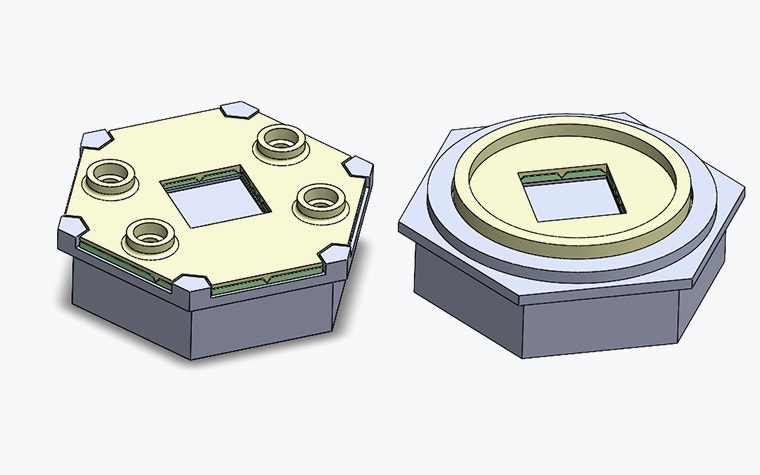

After identifying the final concept, we focused on designing every component in the most cost-efficient way while maximizing quality. Together, we settled on the most suitable manufacturing method and froze key product characteristics.


Satisfied with the outcome, the project moved into series production. The module’s design combined 3D-printed components with off-the-shelf and traditionally manufactured parts, all assembled and controlled by Materialise. From concept to production, 3D printing enabled rapid iterations, user-centered design, and high-precision manufacturing of complex, multi-material components — all while meeting stringent performance requirements for surgical simulation. Now, MMI can order the new module as and when they or their customers need it, regardless of quantity.
It's a great example of what 3D printing brings to medtech; it's not just a production method for end-use parts, but a powerful enabler in innovation and training.
Materialise will support you through every stage of the product development cycle.
Bridge or series, we're here to help
And that includes every step, before and after. We're happy to offer our expertise wherever you need it, from identifying the right solution in the discovery phase, to developing, testing, validating, and manufacturing your chosen solution — including the all-important steps for regulatory approval.
3D printing has so much added value for medtech manufacturers. We're here to help you find it.
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.