PRESS RELEASE
Bring Peace of Mind to Medical 3D Printing with Materialise Certified Solutions
November 26, 2018
Partnerships surrounding Mimics inPrint software deliver broad benefits for patient-specific care
CHICAGO – (Nov. 26, 2018). Materialise NV (Nasdaq: MTLS), a world leading 3D printing solutions provider, is working with partners to remove uncertainty and confusion for hospitals and physicians who are incorporating 3D printing into their diagnostic and surgical planning processes.
Materialise has launched a certification program that allows printer manufacturers that partner with the company to have products tested and validated as being fully compatible with Materialise Mimics inPrint software. inPrint is the first and only software to gain clearance from the FDA to develop 3D printed anatomical models for diagnostic and surgical planning uses. The inPrint software is printer- and material-agnostic, allowing Materialise to develop partnerships and solutions to meet a range of hospital and clinician requirements.
Materialise is working to address potential challenges for health care providers by ensuring their 3D operations are fully compatible and able to meet quality standards for developing and printing patient-specific 3D anatomical models. As the backbone of the 3D printing industry, Materialise incorporates nearly three decades of 3D printing experience and expertise to help medical professionals deliver more personalized patient care.
Stratasys and Ultimaker are the first two 3D printing hardware partners to participate in this program and to have their products tested by Materialise to certify compatibility.
“These partnerships offer health care providers the benefits of our open and flexible 3D printing solutions, while eliminating potential compatibility challenges with third-party hardware providers,” said Bryan Crutchfield, Vice President and General Manager of Materialise North America.
“Hospitals and physicians want the benefits of flexible software tools that are material and printer ‘agnostic,’ but they also require the certainty of knowing that flexibility won’t create compatibility challenges at deployment or in the future. We’re ensuring they can focus on patient care and improving people’s lives by providing compatible point-of-care solutions that meet their needs.”
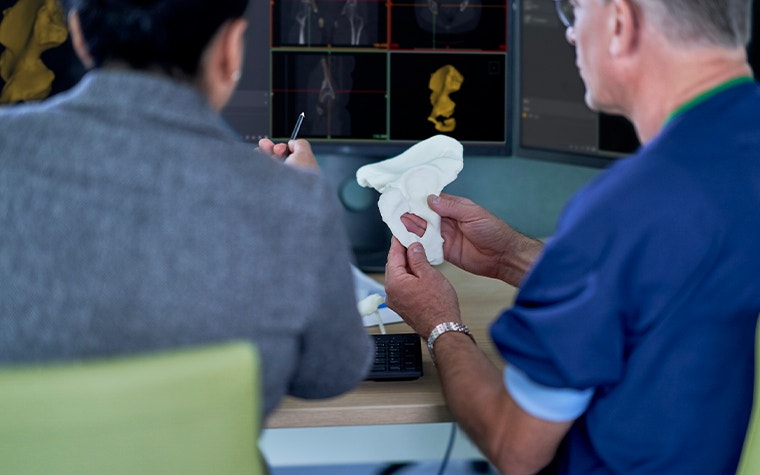

Materialise’s testing certifies that when used in combination with Mimics inPrint software and in compliance with relevant quality assurance standards and in accordance to manufacturer instructions, a printer is capable of printing models suitable for use in surgical planning and multidisciplinary communication.
Attendees at the RSNA Scientific Assembly and Annual Meeting, taking place November 25-30 at McCormick Place in Chicago, can learn more by visiting Materialise at Booth #4114.
More information about the Mimics InPrint Certification Program.

About Materialise
Materialise incorporates 27 years of 3D printing experience into a range of software solutions and 3D printing services, which together form the backbone of the 3D printing industry. Materialise’s open and flexible solutions enable players in a wide variety of industries, including healthcare, automotive, aerospace, art and design, and consumer goods, to build innovative 3D printing applications that aim to make the world a better and healthier place. Headquartered in Belgium, with branches worldwide, Materialise combines the largest group of software developers in the industry with one of the largest 3D printing facilities in the world. For additional information, please visit: www.materialise.com.
Cautionary Statement on Forward-Looking Statements
Some of the statements in this press release are "forward-looking" and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include statements relating to, among other things, our planned commercialization efforts and regulatory approvals of our technologies as well as the success thereof and our research and development projects. These forward-looking statements are based upon the expectations of management under current assumptions at the time of this press release. We caution you that forward-looking statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control that may cause our actual results to differ materially from our expectations. We are providing this information as of the date of this press release and do not undertake any obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or otherwise, unless we have obligations under the federal securities laws to update and disclose material developments related to previously disclosed information.
Materialise press contact
L-102882-01
Share on: