Webinar
Application of Modeling and Simulation in Cardiovascular Engineering
About this webinar
The advanced engineering concepts and complex hemodynamic conditions of blood-carrying medical devices demand new tools to support the development process. Computer-aided simulations and virtual anatomy studies promise to reduce research and development time and costs compared to conventional approaches and give further insights that in vivo and in vitro experiments are not capable of.
The service company enmodes GmbH offers a wide range of assistance and consulting for translational biomedical developments, from small research projects to complex ventures based on clients’ requirements. Starting from computer-supported modeling to production and testing, enmodes GmbH accompanies companies on the road to product maturity.
What you will learn
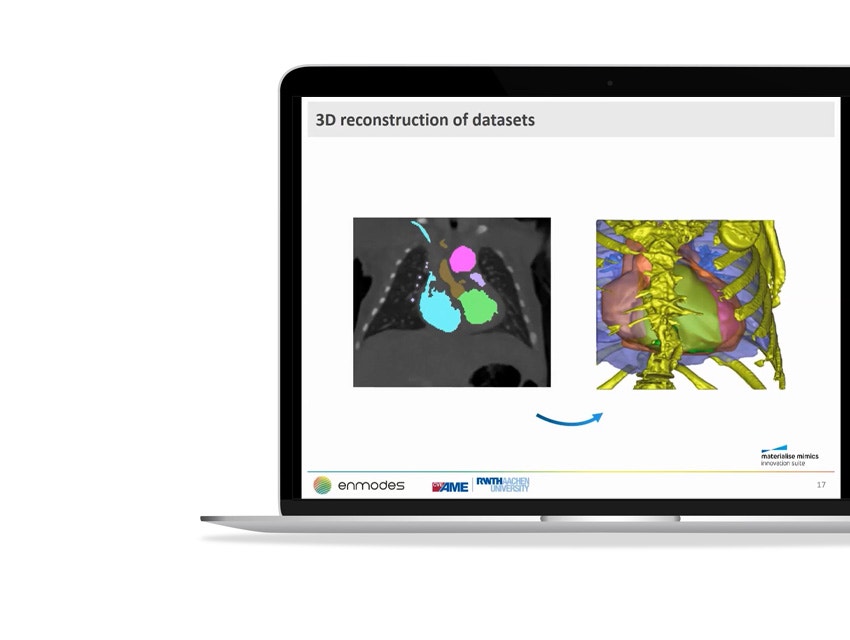
- How enmodes GmbH uses 3D technology to reduce research and development times and costs
- How they use CAD to improve the company's capabilities in the field of blood pumps, oxygenators, and heart valves
Speakers

Dr. Simon Sonntag
Share on:
Materialise medical device software may not be available in all markets because product availability is subject to the regulatory or medical practices in individual markets. In countries where no regulatory registration is obtained of Mimics or 3-matic Medical, a research version is available. Please contact your Materialise representative if you have questions about the availability of Materialise medical device software in your area.
L-100767-01