CUSTOMER STORY
Optimizing Complex Cardiac Device Development

In a competitive transcatheter aortic valve replacement (TAVR) market, one Fortune 500 medical device company is creating innovative solutions for complex cardiac cases. Using advanced 3D modeling tools like Materialise Mimics, the team bridges R&D and clinical applications to ensure precise device planning and improved patient outcomes. This article explores how technology and collaboration are improving care for challenging structural heart conditions.
Challenges in the TAVR market
One Fortune 500 medical device company aims to carve out a niche by addressing complicated cases such as bicuspid aortic valves, failed TAVR or other surgical valves, and aortic regurgitation. Together, these smaller specialties represent a significant number of potential patients globally. A cardiac clinical engineer from the company is passionate about bridging the gap between research and development (R&D) and clinical applications, focusing on challenging cases in structural heart therapy.
Complex cardiac segmentation, surgical planning and more
To tackle these cases, this company leverages the Materialise Mimics software for advanced 3D modeling and printing. The workflow involves segmenting CT scan data using the Mimics AI Heart Segmentation algorithm to create detailed 3D models — virtual and in special cases, physical 3D prints. The anatomic segmentation shows the existing valve structures, the current placement of devices, and potential positioning for a new device. The automation enables the broader clinical engineering team to focus more on detailed elements of the anatomy, along with analyzing and planning the procedure, rather than on the labor-intensive process of segmentation. Next, engineers precisely plan and simulate device placement, helping to ensure optimal outcomes even in the most challenging scenarios.
Case example: valve-in-valve procedure
One illustrative case involved a patient with a failed valve that had embolized into the aortic arch, requiring a replacement valve.
“The challenge was to deploy our valve within this constrained anatomy without compromising hemodynamics or coronary flow,” explained the cardiac clinical engineer. “Using Mimics, the team created a detailed 3D model to visualize and plan the procedure, then 3D printed the anatomy to test the valve deployment physically. This meticulous planning ensures that the valve is positioned correctly, avoiding coronary obstruction and enabling good leaflet function. Using Mimics, the cardiac team has been able to ramp up from one to two cases a month to 10 a week.”
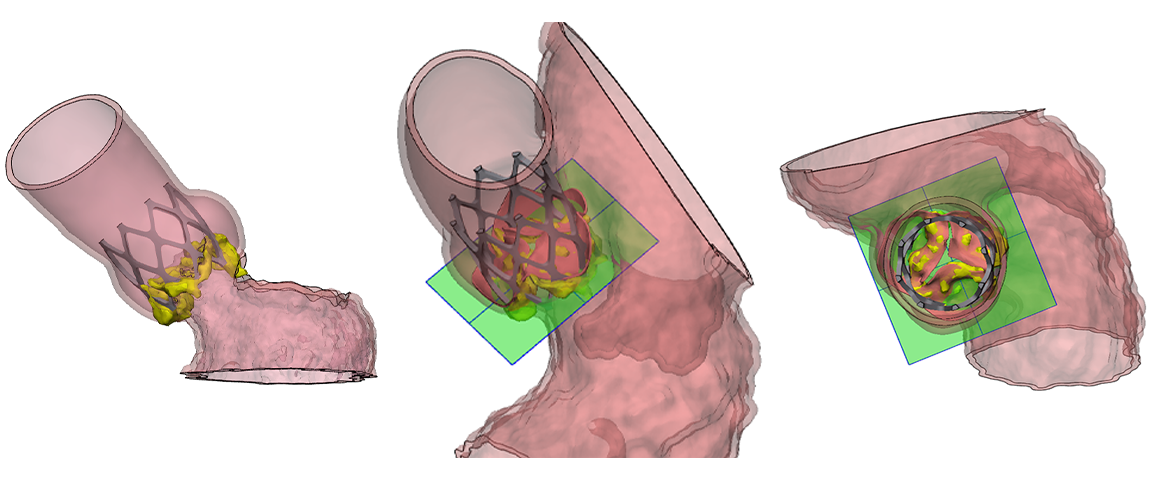
An example 3D left heart model was created from a contrasted CT scan, allowing clear visualization of the aortic valve and precise device planning based on patient-specific anatomy.
The feedback loop that accelerates device design
An essential aspect of this device design workflow is the feedback loop in which clinical information from the procedure is provided back to the clinical engineering team. This continuous knowledge stream helps the team refine their models and simulations, ensuring that future cases benefit from the lessons learned. By aggregating data from various cases, the team can identify patterns and improve their devices' design and deployment strategies. This real-time clinical insight is invaluable for ongoing R&D efforts, with the goal of making the devices more effective and safer for patients.
“The use of Materialise Mimics software has been instrumental in enabling our company to address these complex cases efficiently and effectively. By providing detailed 3D models and simulations, we can offer precise guidance and support to our R&D team, helping to ensure better patient outcomes in the future.”
Materialise medical devices may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Materialise representative if you have questions about the availability of Materialise Medical devices in your area. Mimics Research cannot be used for clinical applications.
L-104451-01
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.