Webinar
Mimics Innovation Course on Cardiac 3D Reconstruction: Making the Complex Simple in Conjunction with King’s College London
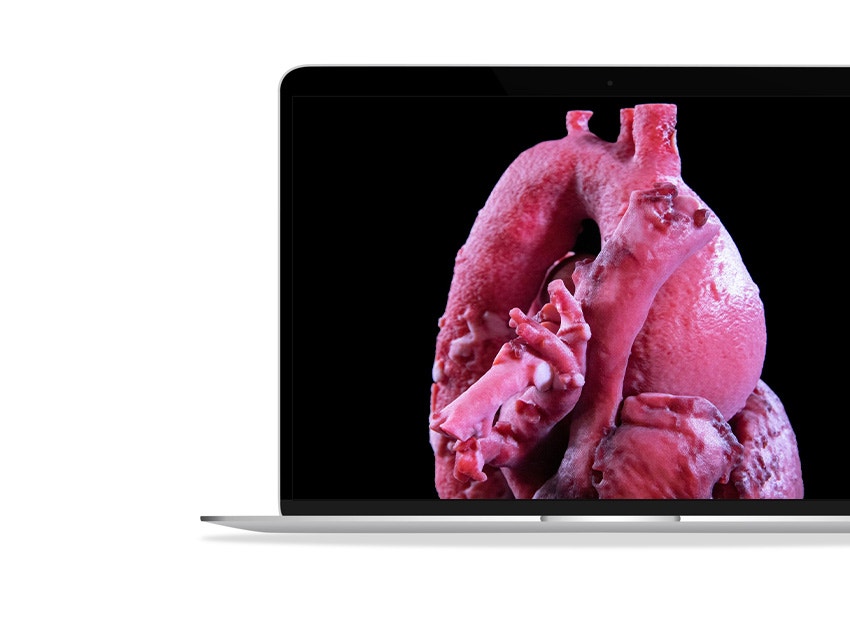
About this webinar
Would you like to be taught by experts in cardiac surgery on the latest advancements in 3D technology for surgical planning? Alongside King’s College London, world-class clinicians presented their cases for adult and pediatric cardiac reconstruction and how they have made previously untreatable cases simple.
There were tips and tricks, a Q&A, and live demonstrations of how to get the most from the Mimics Innovation Suite. Materialise application engineers were also on hand to show optimized workflows so time can be spent most efficiently preparing for complex cases. This course has been approved by the CPD certification service.
What you will learn
- The fundamentals of setting up a 3D planning service for congenital heart disease in a hospital
- How 3D planning adds value to clinical cardiac applications
- The challenges of segmentation and how to overcome them
- Future perspectives for optimizing 3D planning as part of the patient pathway
Speakers

Dr Giovanni Biglino

Dr Greg Skinner MB BS MRCPCH

Dr. Saravanan Durairaj MB BS DCH MRCPCH

Dr. Peter Metherall

Dr Kuberan Pushparajah

Mr. Nick Byrne
Share on:
Materialise medical device software may not be available in all markets because product availability is subject to the regulatory or medical practices in individual markets. In countries where no regulatory registration is obtained of Mimics or 3-matic Medical, a research version is available. Please contact your Materialise representative if you have questions about the availability of Materialise medical device software in your area.
Materialise medical device software may not be available in all markets because product availability is subject to the regulatory and/ or medical practices in individual markets. In countries where no regulatory registration is obtained of Mimics and/or 3-matic Medical, a research version is available. Please contact your Materialise representative if you have questions about the availability of Materialise medical device software in your area. L-102307